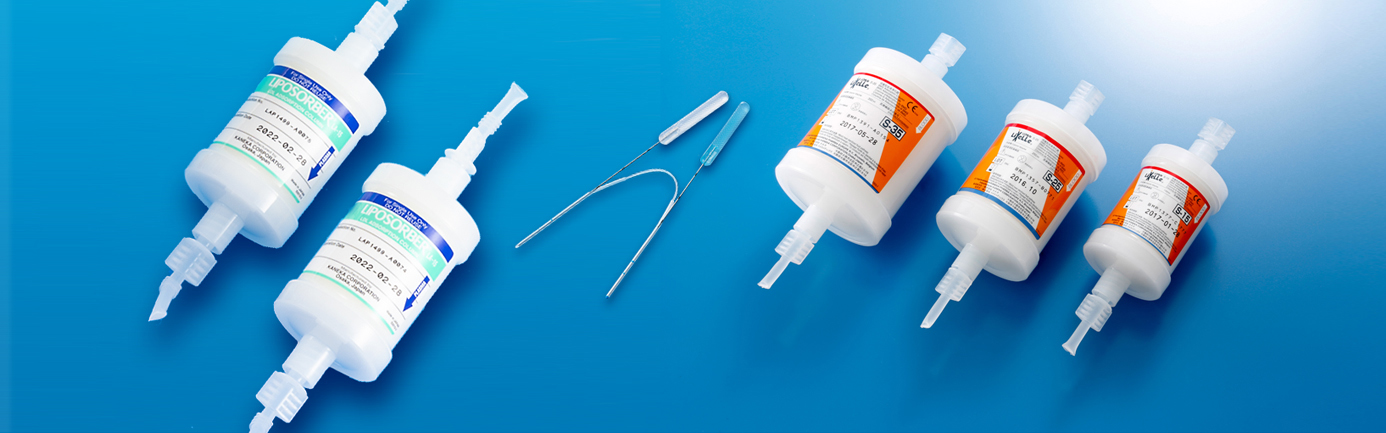
Products
LIPOSORBER®
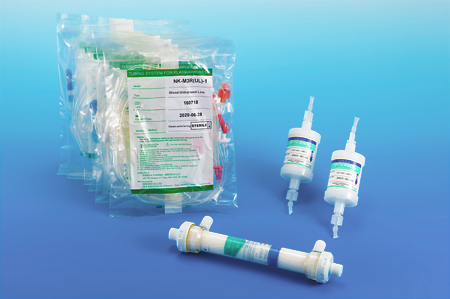
Clinically Diagnosed Familial Hypercholesterolemia (FH)
The LIPOSORBER® system is indicated for patients for whom at least 6 months of diet and maximum drug therapy has been either ineffective or not tolerated, along with the following criteria:
- Group A – Clinically diagnosed Familial Hypercholesterolemic Homozygotes with LDL-C >500 mg/dL;
- Group B – Clinically diagnosed Familial Hypercholesterolemic Heterozygotes with LDL-C ≥300 mg/dL;
- Group C – Clinically diagnosed Familial Hypercholesterolemic Heterozygotes with LDL-C ≥100 mg/dL and either documented coronary artery disease or documented peripheral artery disease.
- Group D – Clinically diagnosed Familial Hypercholesterolemic Heterozygotes with LDL-C ≥ 100 mg/dl and lipoprotein(a) [Lp(a)] ≥ 60 mg/dL, and either documented coronary artery disease or documented peripheral artery disease.
Focal Segmental Glomerulosclerosis (FSGS)
The LIPOSORBER® system is indicated for use in the treatment of adult and pediatric patients with nephrotic syndrome associated with primary focal segmental glomerulosclerosis, when standard treatment options, including corticosteroid and/or calcineurin inhibitors treatments, are unsuccessful or not well tolerated and the patient has a GFR≧60 ml/min/1.73m2 or the patient is post renal transplantation.
LIPOSORBER® for FSGS - Safety & Probable Benefit >>>LIXELLE®

LIXELLE® Beta 2-microglobulin (β2M) Apheresis Column is indicated for the treatment of patients with clinically diagnosed dialysis-related amyloidosis (DRA).
LIXELLE® for DRA - Safety & Probable Benefit >>>LIXELLE® for DRA - Instructions for Use >>>
LIXELLE® for DRA - Patient Guide >>>
LACRIFLOW® CL
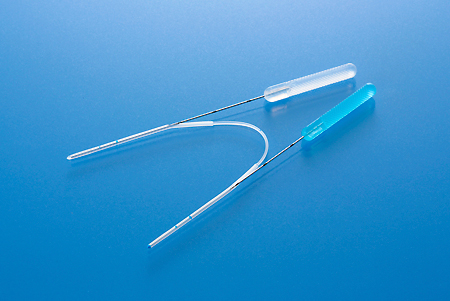
LACRIFLOW® CL is indicated for the treatment of epiphora in patients 12 months and older, in case of:
- Canalicular pathologies (stenosis, obstruction, lacerations),
- During Dacryocystorhinostomy (conventional or laser),
- Congenital nasolacrimal duct obstruction